DDI-Explorer
Coming soon! Pre-order available!
Overview
The concurrent administration of two or more medicines may result in interaction between their components. Such interaction might be of minor, moderate or serious impact on the health condition of patients. 2 – 3% of drug-drug interactions (DDI) reported at a worldwide level has led to life-threatening, and even fatal, consequences. 14 – 16% of the preventable medications error in the US was attributed to mistaken combination of drug substances. Reasons for this state-of-affair in this matter are too numerous to count. Clinical practioners could not be held responsible for this matter since evidence on the interaction might not have been available at hand when medicines were prescribed to their patients. Such practioners are inclined to blame it on the drug information databases for their inability to avail ready access to relevant information on DDIs matters. In a recent survey in Washington DC area, 510 pharmacy shops, employing DDIs software, were asked to identify the likely interactions in seven dummy prescriptions. These systems failed to identify existing DDIs in about 33% of the case despite the fact that official generic names were used to issue the dummy prescriptions. Such performance rating could have been worse if brand names and/or multiple ingredients products were contained in the dummy prescriptions. Controversy on the reason for such shortcomings is still waging. While some put the blame on the information databases, others blame the efficiency of computerized DDIs systems.
Information pertaining to DDIs is scattered in more than a dozen drug databases. These include the internationally reputed Martindale Extra Pharmacopoeia (Dale), the American Hospital Formulary Service (AHFS), the United States Pharmacopoeia Drug Information (USP-DI), the German Pharmacopoeia and other DDIs specific publications such as Hartman Ammon, Philip Hansten and Evan Stockley. The sources are characterized by the lack of overlap in the body of information they offer. The diagram provided hereunder highlights the overlap between 11,260 interactions reported USP-DI and other databases. Whilst the bracketed values in the below diagram represent the number of DDI reports contained in each database, the values in RED represent the frequency of overlaps.
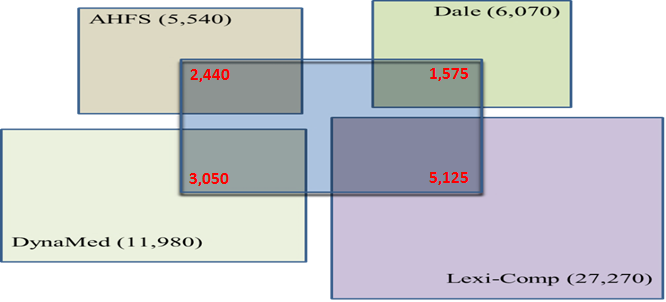
While the signification of the above argument are self-evident, they distinctly underline one of the most serious reasons for prescribing error that could be otherwise detected by healthcare professionals.
Some of other equally serious deficiencies in reporting DDIs may be related to the inadequate identification of DDIs caused by induced drug effects despite the fact that such effects are extensively documented by regulatory agencies.
In addition, the independent, and often inconsistent, reporting of DDIs related to drug and their prodrugs adds extra insult to the injury. For example, some of the life-threatening interactions for Amphetamine and the amphetamine class of drugs are provided in most of the databases. Notwithstanding, DDIs for a dozen of prodrugs and/or precursors of amphetamine or monoamines, are almost totally unreported by such databases.
The DDI-Explorer has emerged with the promise of accounting for all of the above mentioned shortcomings. With more than two decades of dedicated search in the interaction literature, our system presently provides information based on more than 125,000 interaction reports that have been documented in more than a dozen of reputable information databases.
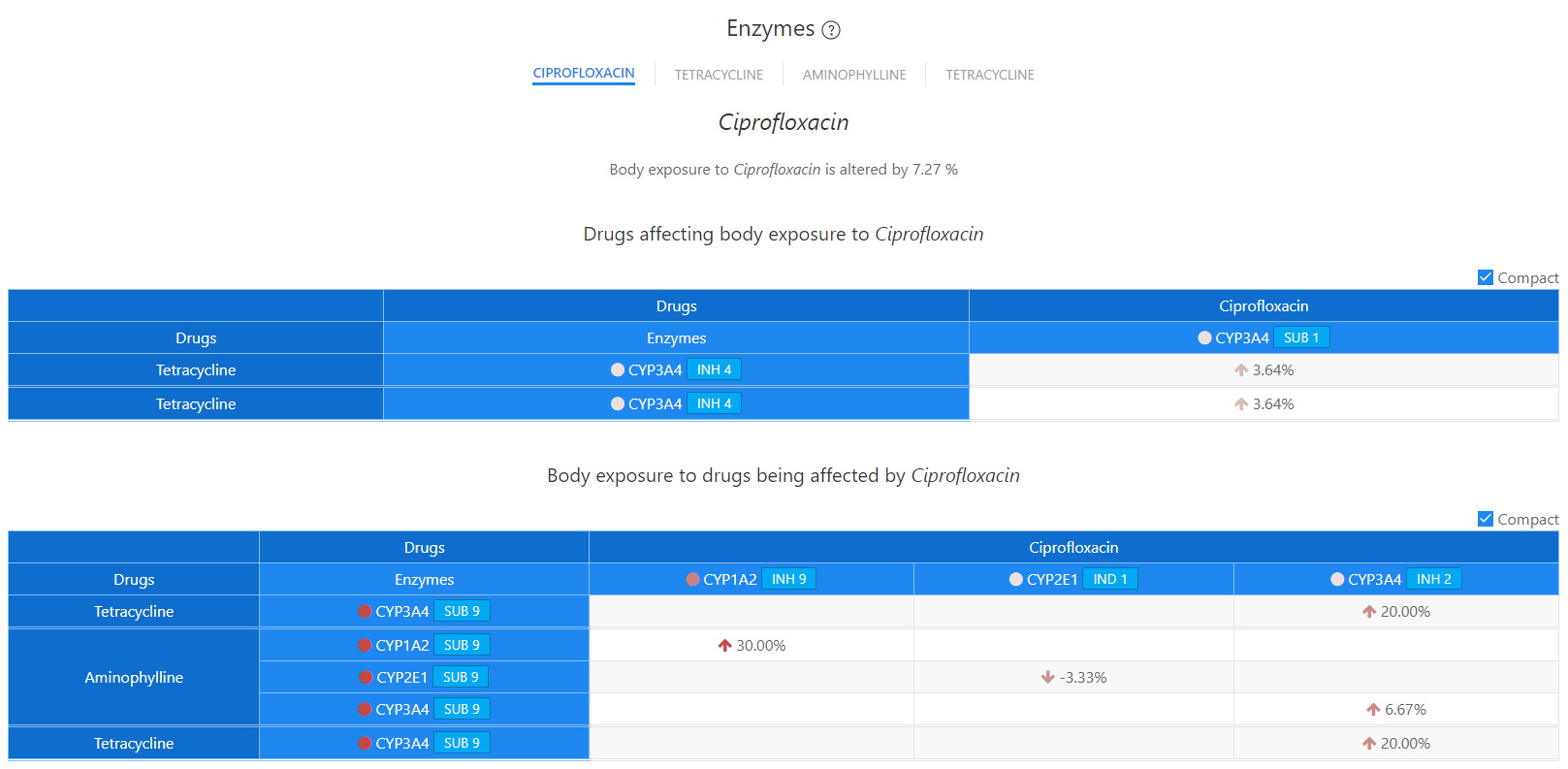
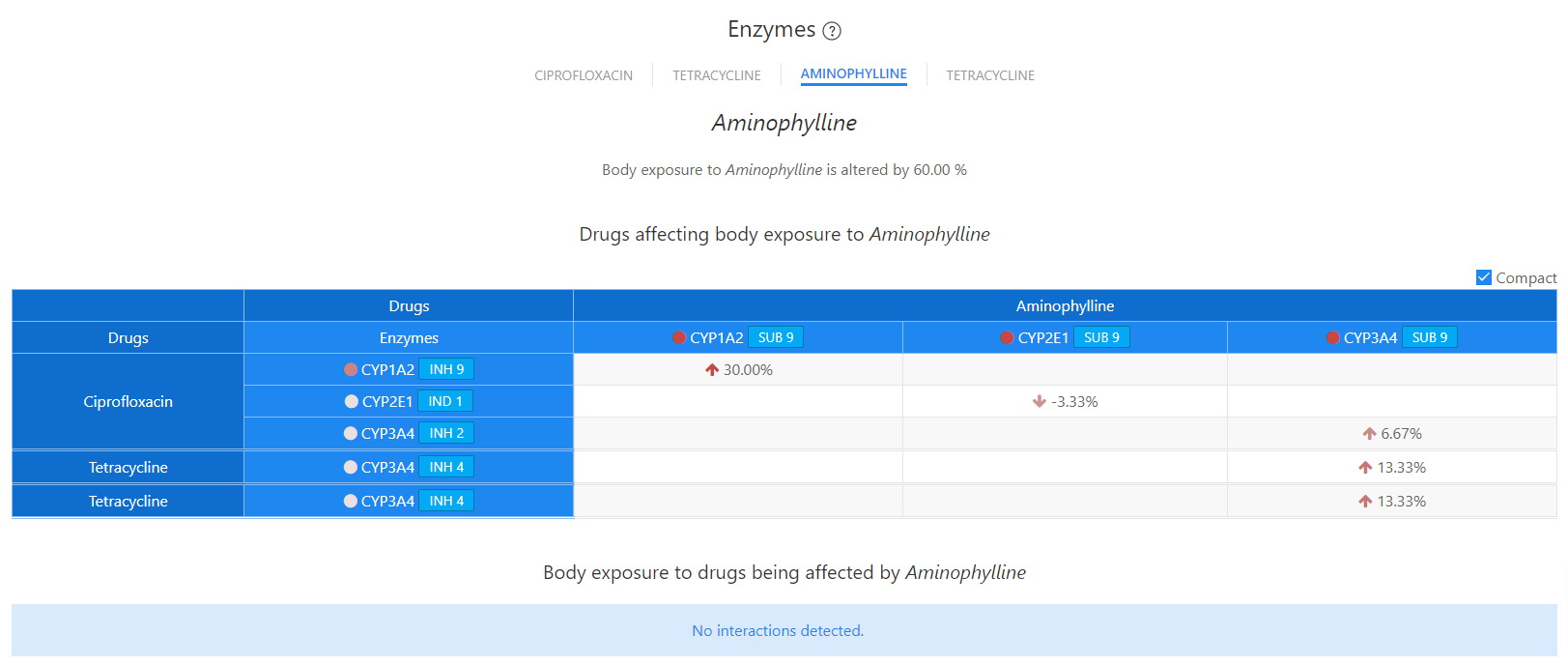
DDI-Explorer has been supplemented with some unique features that exactly identify the kinetic or dynamic bases for a reported interaction. The implication of liver enzymes and transporters in reported interactions is precisely and concisely provided by the liver and target matrices. Such a tool would prove to be indispensable for practitioners and researchers since it would enable them to predict interaction not reported the relevant literature.
Unlike other databases, the Liver Enzymes / Transporter modality of the DDI-Explorer offers a unique and novel tool that disambiguates the generalized and incomplete reporting of the implication of liver enzymes in DDIs.
The DDI-Explorer is intended for use by professional individuals (pharmacists, physicians, nurses) and corporate establishment such as hospitals, clinics, healthcare institutions and Pharmaceutical industry). It could be also used by ordinary individuals who strongly advised NEVER to change their medicines without consulting with their healthcare professionals.
The DDI-Explorer gives easy online access to the whole database and can be used on your desktop PC, notebook, smartphone, tablet PC and even Smart TV – any device that has a browser and access to the Internet. Mobile apps coming soon!
For further details on the above mentioned arguments, users are requested to consults the author’s review on the topic in the Wiki area here.
Screenshots
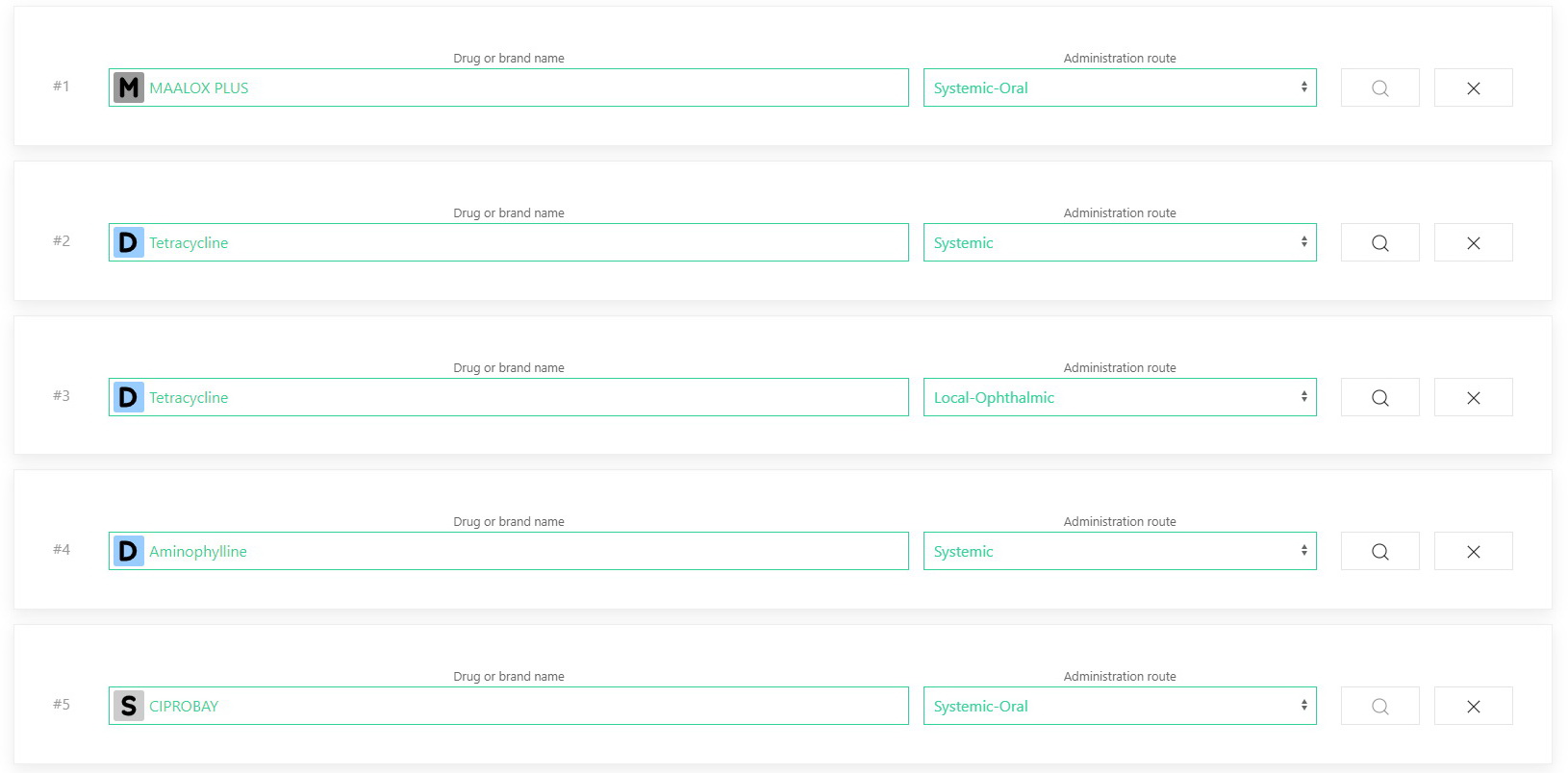
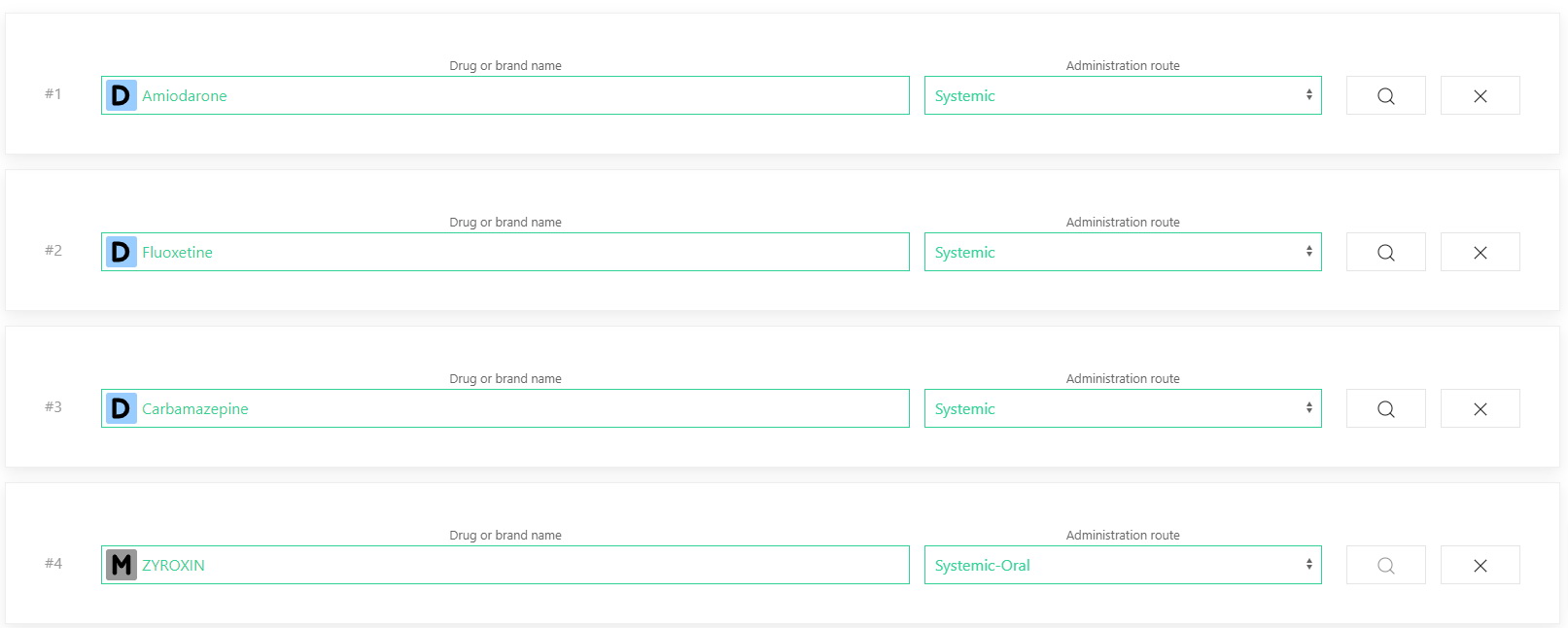
Drugs to be analyzed
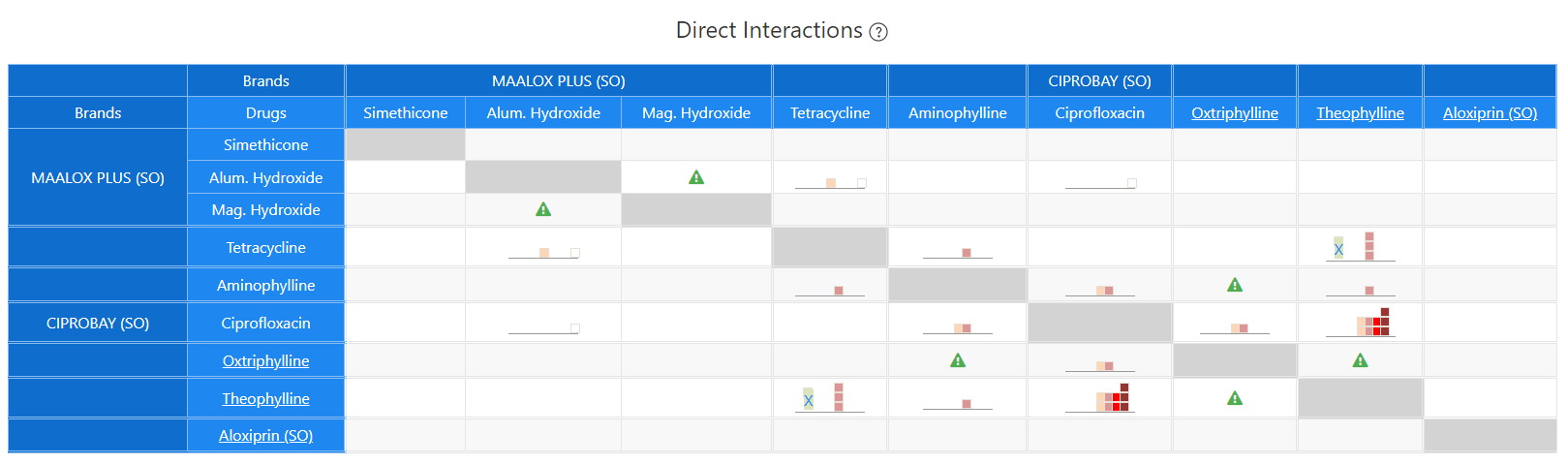
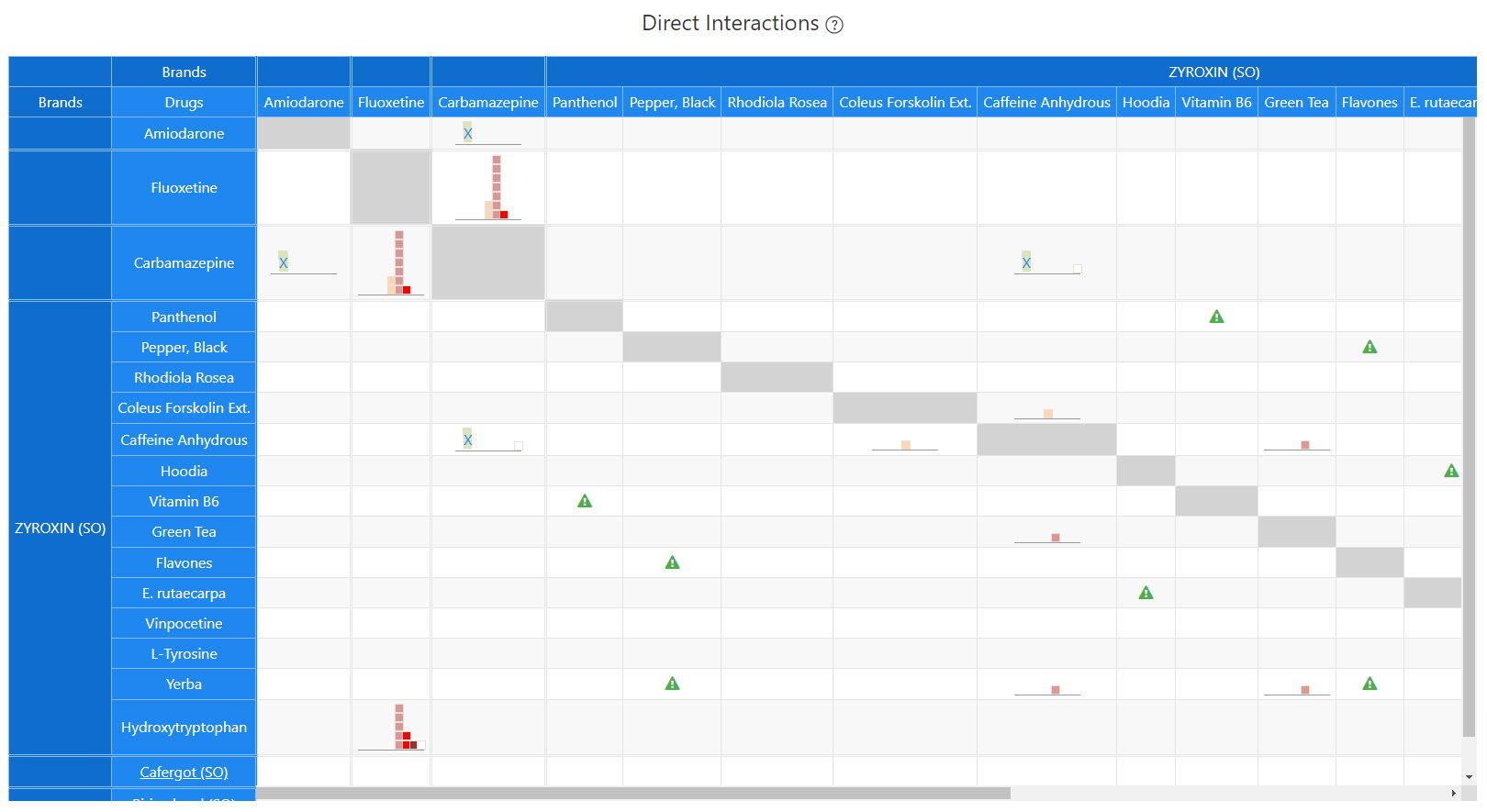
Direct Interactions
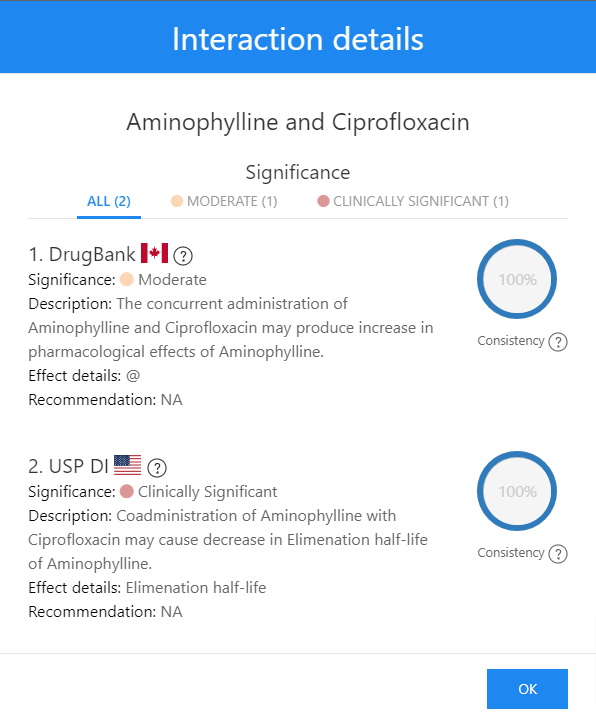
Ciprofloxacin and Aminophylline Interaction
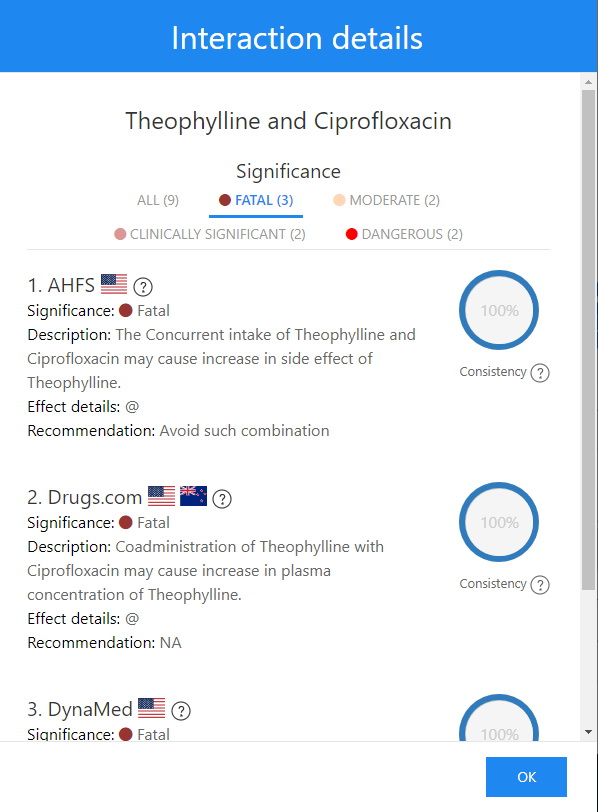
Theophylline and Ciprofloxacin Interaction
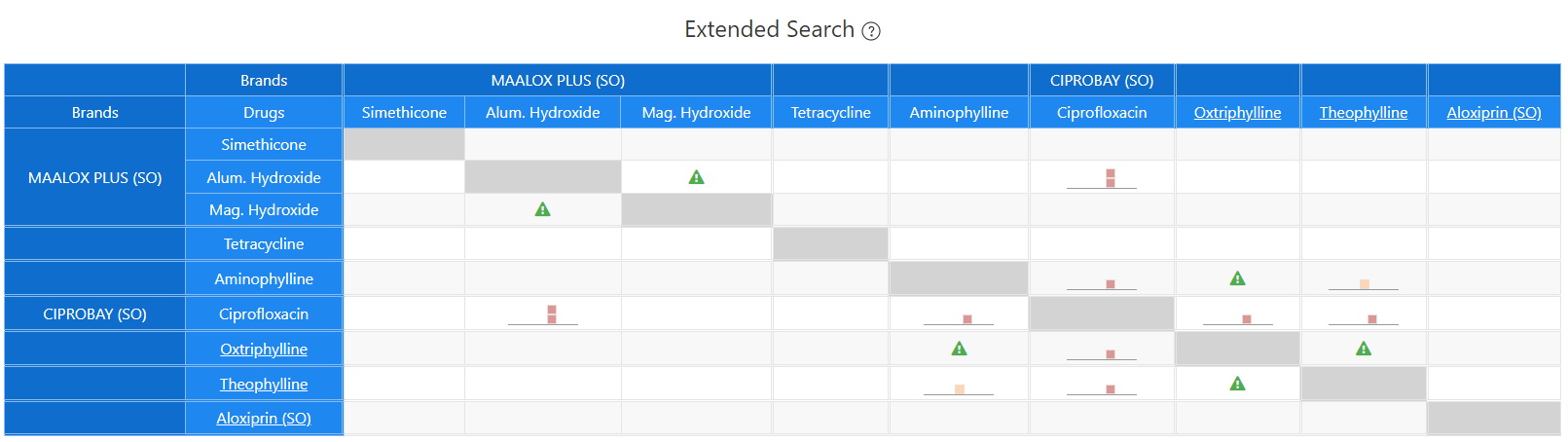
Extended Search
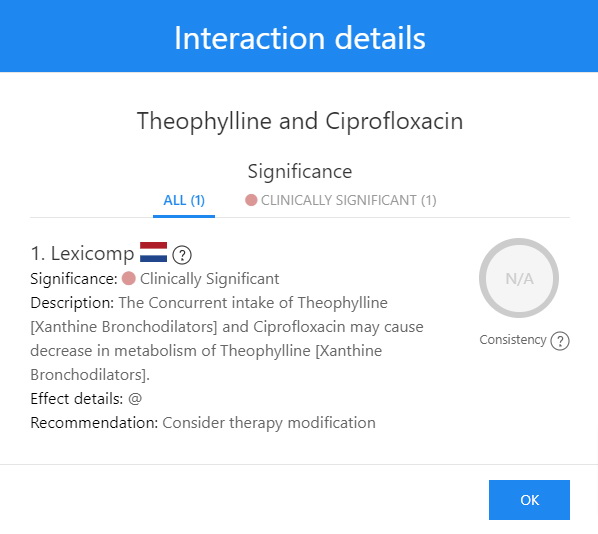
Theophylline and Ciprofloxacin Extended Interaction
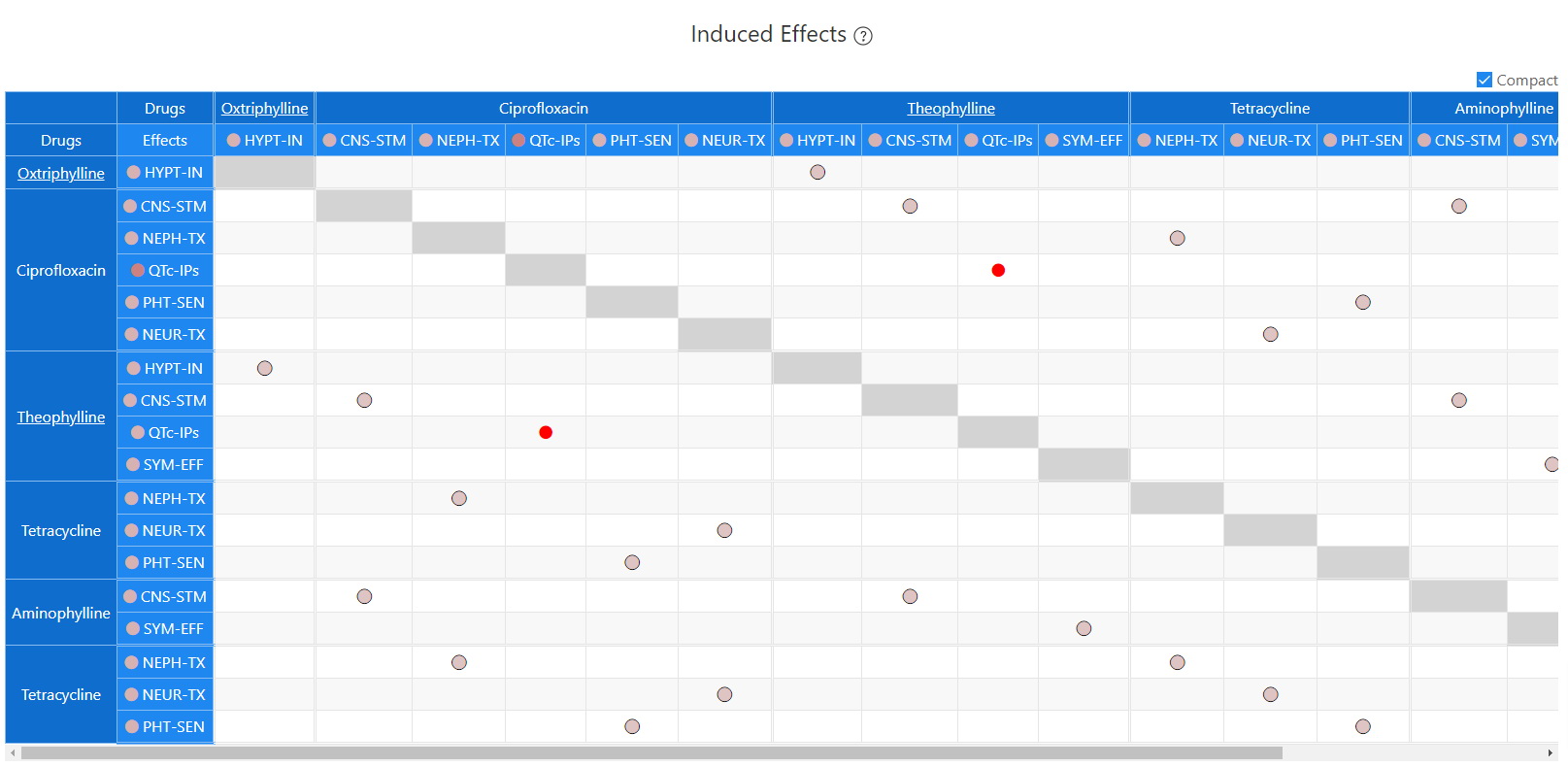
Induced Effects
Enzymes - Ciprofloxacin
Enzymes - Aminophylline
Drugs to be analyzed
Direct Interactions
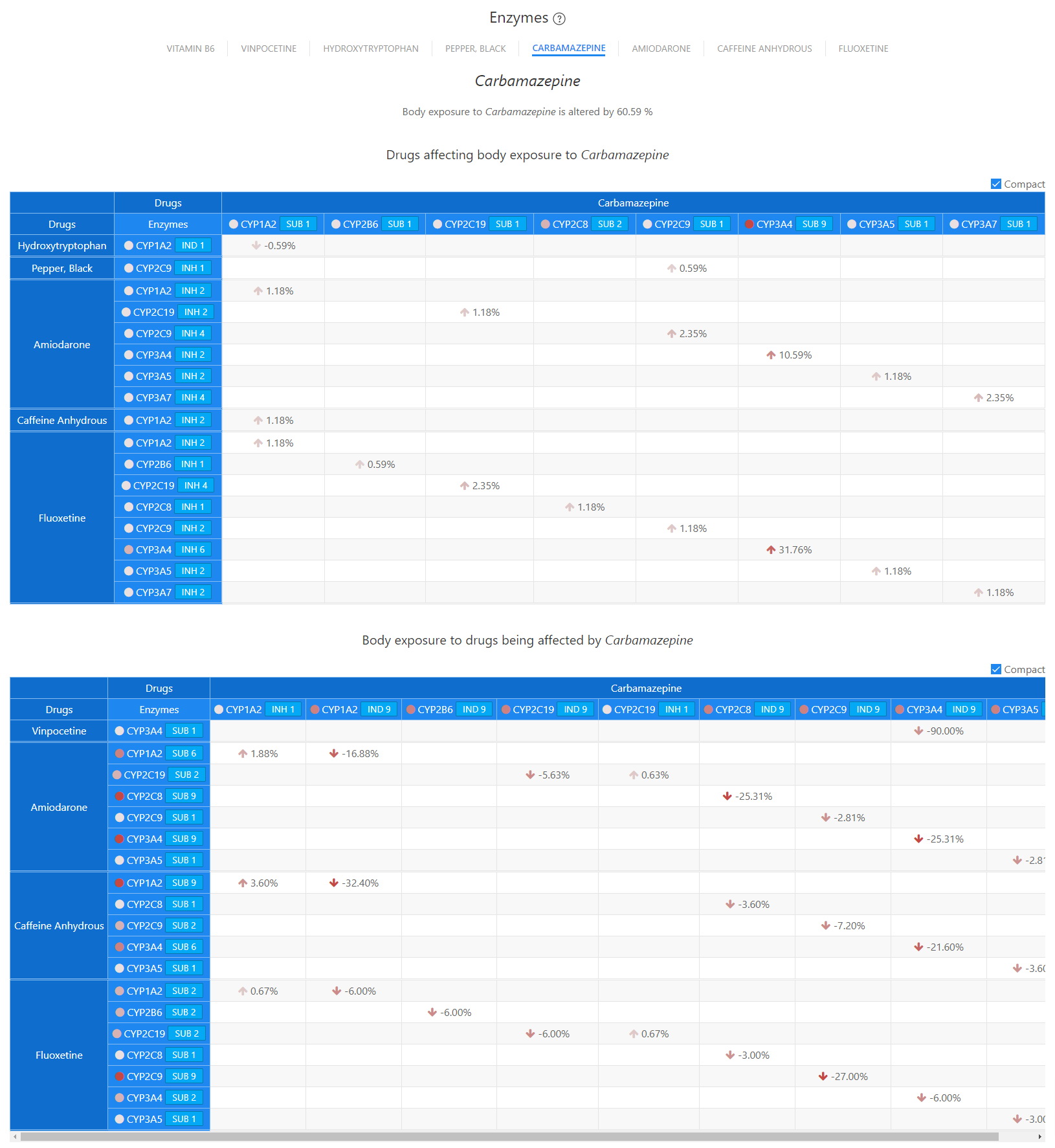
Enzymes - Carbamazepine
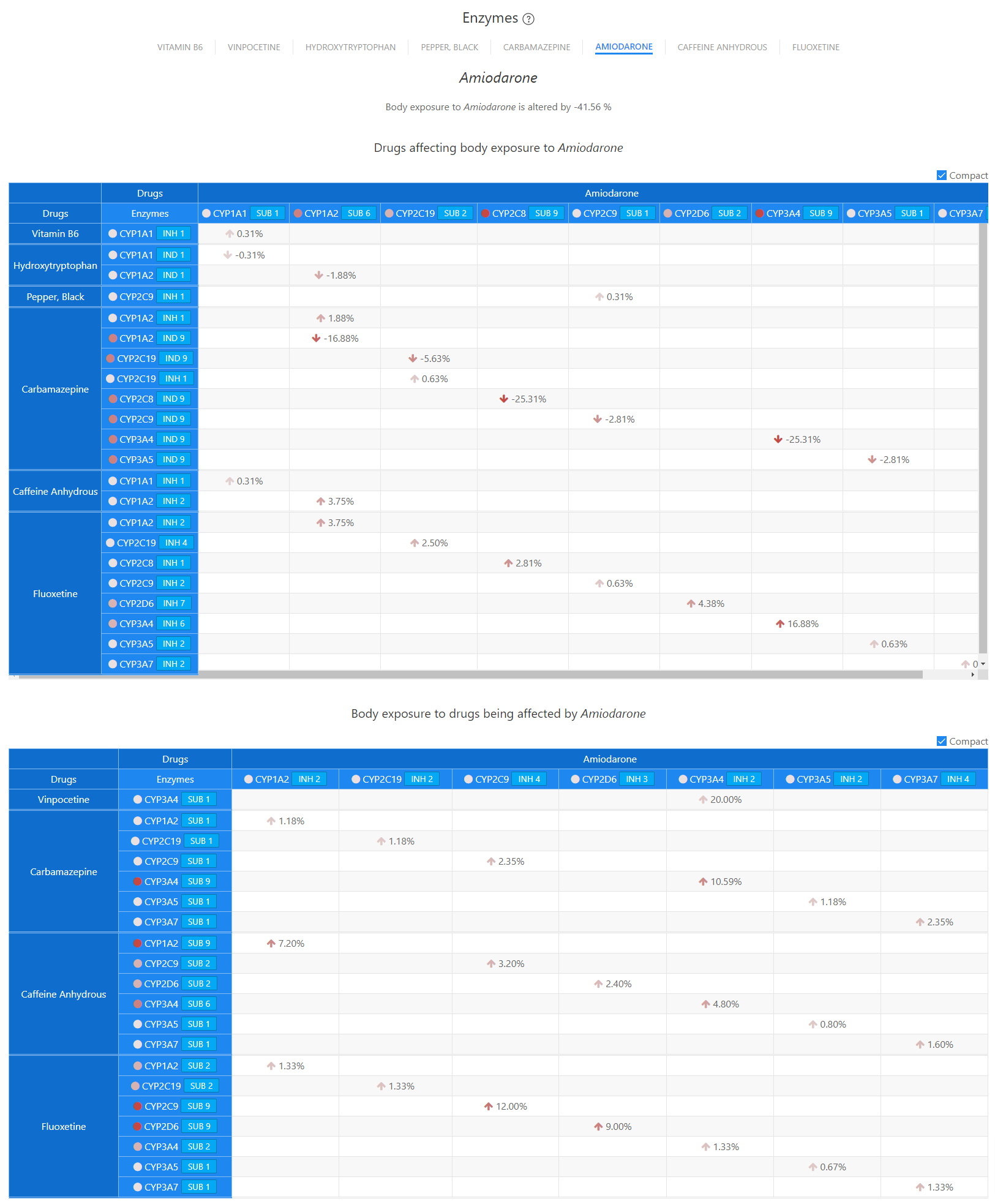
Enzymes - Amidodarone
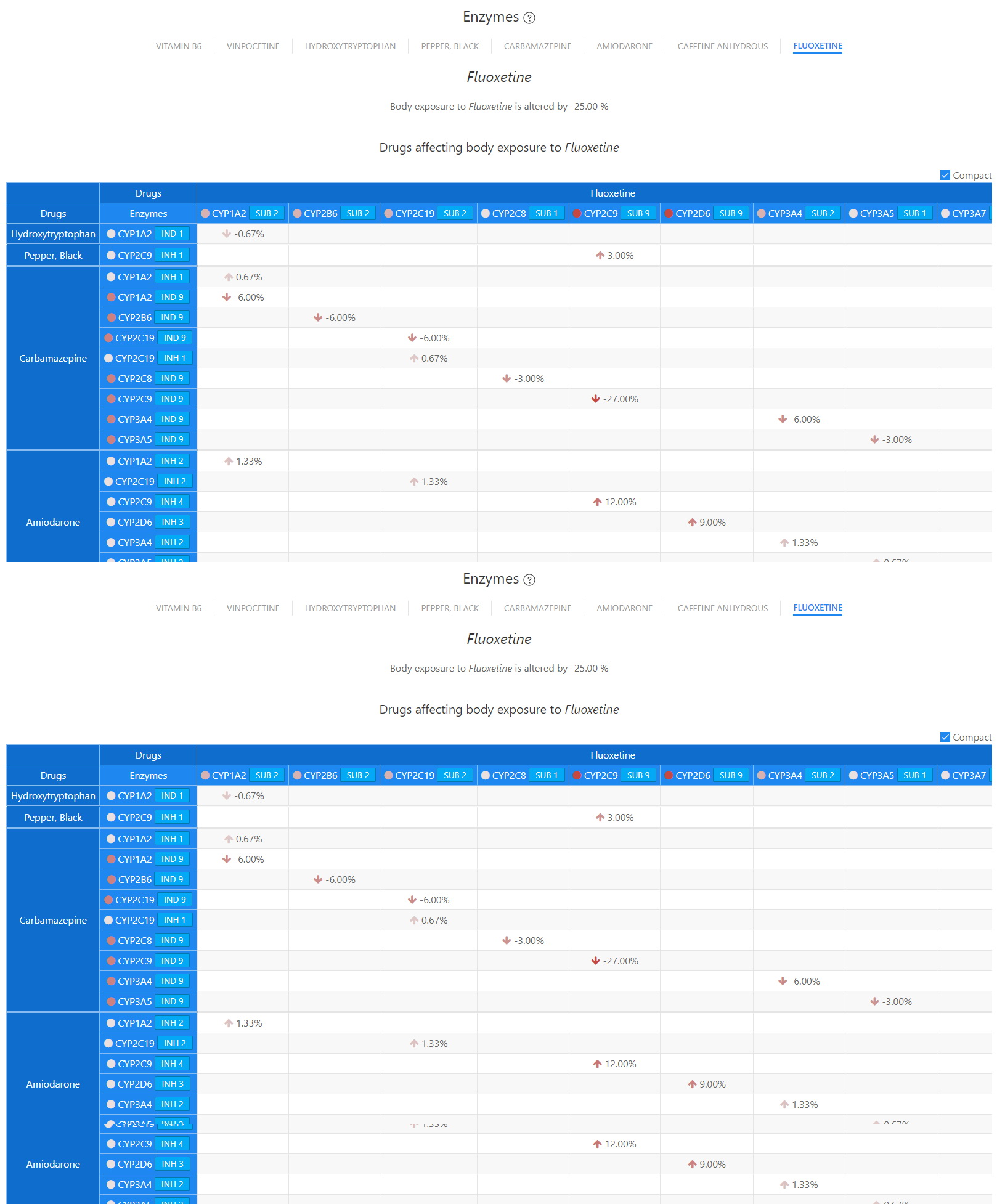
Enzymes - Fluoxetine
Videos
Screencasts of the product, coming soon.
Features
- Single or multiple drugs interactions, you may choose up to 50 drugs for simultaneous interactions analysis
- Drugs interactions information based on top up-to-date 12 world-wide databases from 6 countries: The American Hospital Formulary Service (AHFS, USA), Hartman Ammon (Germany), The British National Formulary (BNF, GB), DataBank (dBank, Canada), Drugs.com (USA, New Zealand), Facts & Comparisons (F&C, USA), Philip Hansten (USA), Martindale (GB), Linfo (Sweden), The Rota Liste (Germany), Ivan Stockley (GB), USP-DI (USA).
- Cross-database search and analysis, complete and unprecedented Evidence-Based information.
- 100% transparent and verified results: you get information about the drugs interactions and how they are listed in the world-wide databases and may check (verify) it if you want.
- Support a huge number of international and national generic and brand drug names, to make sure you can find information about interactions no matter who is the supplier/manufacturer of the drug or country of origin
- Works on all devices (desktop PC, notebook, smartphone, tablet PC, etc.)
Benefits
Why do you need DDI-Explorer?
For personal use:
- Verify that your medicine prescription is safe and can be used with other drugs
- Get information about a single drug
For professional use:
- Verify that your medicine prescription is safe and can be used with other drugs
- Get information about drugs and their components, what drugs can be replaced
- Eliminate medical prescription errors and negative side-effects
FAQ
General questions
Can drug interactions be really dangerous?
Yes, absolutely. According to statistical data of the USA about 14 - 16% of human and resources losses are the cause of preventable medications errors (PME) that cost the US 176 Billion Dollars (1995). Besides, 2.8% IAs resulted in fatalities, 14.5% IAs were life- threatening.
What is the generic and brand drug names?
Each medicine has an approved name called the generic name. A group of medicines that have similar actions often have similar-sounding generic names. For example, phenoxymethylpenicillin, ampicillin, amoxicillin and flucloxacillinare in one group of antibiotics. Many medicines also have one or more brand (trade) names. This is chosen by the company that makes it. Several companies may make the same generic medicine, each with their own brand name. The name is often chosen to be memorable for advertising, or to be easier to say or spell than the generic name. For example, paracetamol is a generic name. There are several companies that make this with brand names such as Panado®, Calpol®, etc.
How does DDI-Explorer work?
The DDI-Explorer gives results based on our "know-how" algorithms of cross-databases information processing, so that we give precise and most complete information about drugs and their dosage.
Is DDI-Explorer reliable?
The DDI-Explorer is a reliable and trustworthy tool because it uses transparent safe processing algorithms and up-to-date information from world-wide drugs databases (AHFS, Stockley, MARTINDALE, USP-DI, F&C, etc.). The DDI-Explorer has access to more drugs databases than any other tool on the market. More scientific information available at wiki area.
Usability questions
Can I use DDI-Explorer without Internet?
The full access to complete version of DDI-Explorer requires Internet. However we're planning to make compact versions of DDI-Explorer as smartphone apps that may work without Internet.
Do you have a trial or demo access to DDI-Explorer?
Generally, we do not give a trial access. You may get a complete information about our product using screenshots, demo videos, texts, presentations and information at the wiki area. Our managers are pleased to make live presentations for you, consult and answer all your questions. Please, fill in the contact form and we will assign a personal manager for you.
Pricing and license questions
Does the DDI-Explorer functionality depend on pricing plan?
No, the DDI-Explorer provides the complete full functionality no matter what pricing plan you use. The difference between the pricing plans is only in the support features.
Can I use the DDI-Explorer for my work?
Yes, you can. The DDI-Explorer gives reliable results and is safe to use at your professional activities. However, you need to apply for the professional license. Besides, your company may apply for enterprise license that is more suitable for organizations.
Can I apply for personal license?
You can apply for the license if you're going to use the product as individual for personal (or family) use. You need professional license if you work as a doctor, nurse or in medical or healthcare company (clinic, hospital, drug store, etc.) or you plan to use it at your work or business (e.g. consulting company, law company, etc.).
Do you have any discounts?
Sometimes we make discounts, e.g. for Christmas or Black Friday, but it's not a general rule and may vary. We have flexible pricing plans that already include price reduction in higher plans. We have special offers for our existing clients for subscription renewals; please contact your personal manager for more information.
Reviews & feedback
Coming soon.
Software & hardware requirements
The DDI-Explorer is distributed on SaS model, so everything you need is a modern browser (Chrome, Firefox, IE, Safari, etc.) and Internet to access the product. It doesn't matter what operating system you use (Windows, Linux, iOS, Android, etc.). After you purchase the license, you will get a link and one or more username/password for authenticated access. You may use any device desktop PC, notebook, smartphone, tablet PC, etc. The DDI-Explorer works behind proxy-servers, so there will be no problems accessing it in corporate environments with strict security policies. There are no requirements for the Internet channel, you may use Wi-Fi, wired LAN connection, satellite Internet or mobile (2G, 3G, 4G, etc.).
Clients
Among our clients that use the DDI-Explorer are:
- Ministry of Healthcare of Russian Federation (pre-order)
Wiki
More information about the concept of DDI-Explorer, scientific results and explanations, statistical data and presentations available at wiki area.
Support
We provide support services for our clients according to their pricing plans. The support is available via ticket system (coming soon), online chat (coming soon), support form and phone via call-center (coming soon).
Pricing and licenses
Currently the product is at final testing stage. Please, fill this form for pre-order to be the first who will gain access to the DDI-Explorer. You will get special pricing and offers when the product goes live. We will assign personal manager for you who will inform you about the development details and help you pre-order/buy the product.